Phase Changes
Tuesday, 29 May 2018
1:28 PM
- When a substance changes form
- A substance can have no change in its temperature when given heat
- The energy required to effect the change is known as the Latent Heat
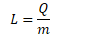
- A phase change requires a 'nucleation site' (a disturbance)
Q
- energy to change the phase (J)
m - mass (kg)
Use when there is no phase change![]()
Use when there is a phase change![]()
Energy into a system = ![]()
Energy out of a system = ![]()
As a consequence, if a disturbance does not occur, a substance (ie water) can stay in its current phase - ie supercooling and superheating
(Ie you can have water in its liquid
state below that will only turn into ice if you quickly thump it)![]()