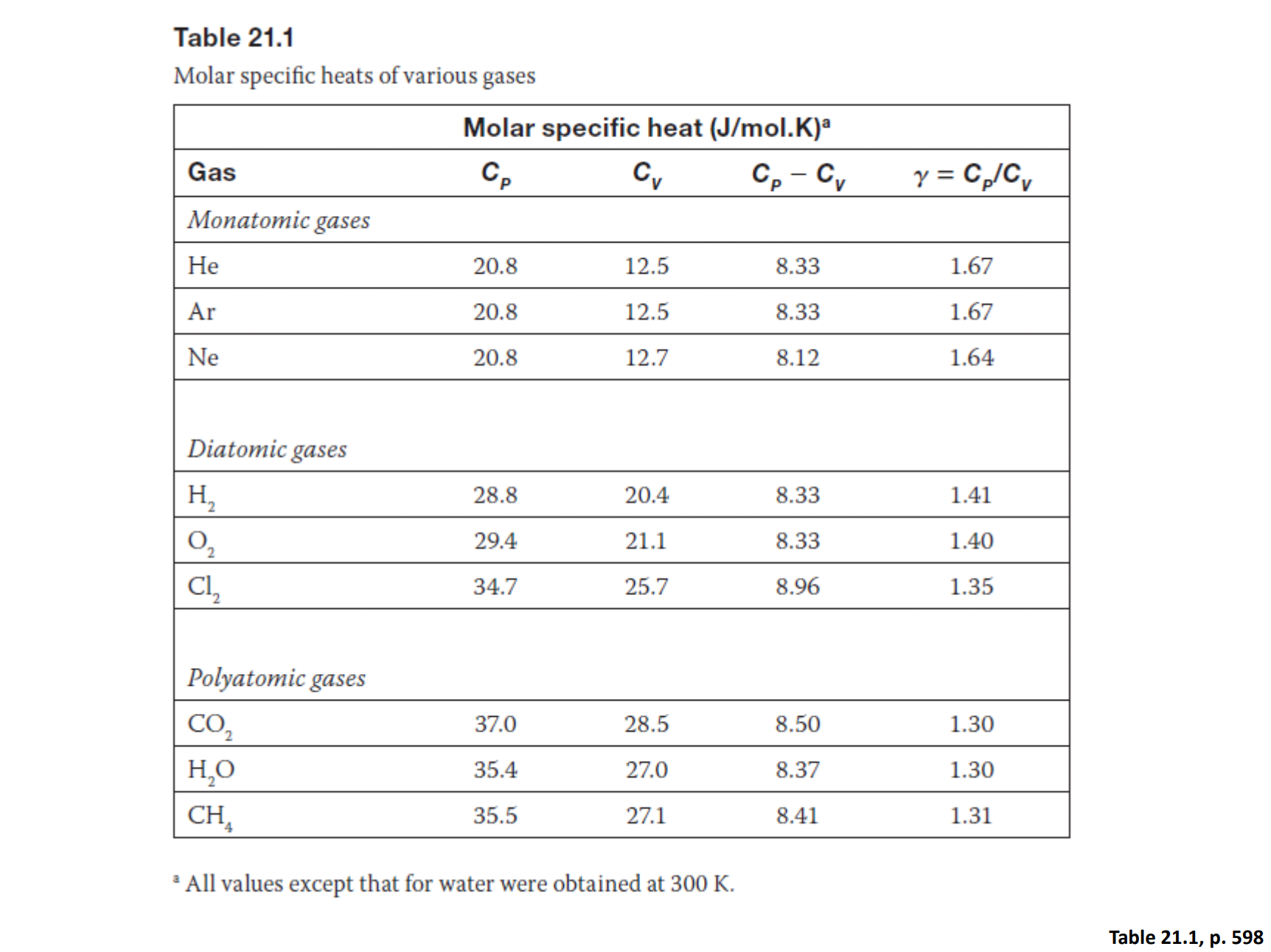
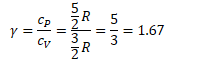Molar Specific Heats
Monday, 7 May 2018
1:29 PM
Gasses also have specific heat properties - and are defined as the amount of heat needed to raise one mole of a gas by 1K
![]()
Where
-
heat required![]()
-
number of moles of gas![]()
-
specific heat of the gas![]()
- change in temperature (final - initial)![]()
But wait, the molar gas constant isn't constant!!!?!?!?
At constant volume -
![]()
At constant pressure -
![]()
Calculating the Specific Heat under Constant Volume
- Constant volume means that no work is done on or by the gas.
Recall the first law of thermodynamics - ![]()
![]()
![]()
![]()
Calculating the Specific Heat under Constant Pressure
Recall the first law of thermal dynamics - ![]()
![]()
Specific Heat Relation
![]()
General Specific Heat Ratio
![]()
Ie for monatomic gases (three degrees of freedom)
![]()
![]()