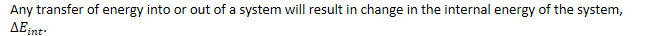Heat
Monday, 7 May 2018
12:52 PM
Heat is defined as the transfer of energy across the boundary of a system due to a temperature difference between the system and its surroundings.
Heat is transferred from the body at the higher temperature to the body at a lower temperature.
Units = Joules (J)
1 calorie = 4.186 J
(1 food calorie = 1000 calories = 1kcal = 4.186kJ)
----
- Energy (in a system) is always conserved
- Any transfer of
energy into or out of a system will result in change in the internal
energy of the system, .