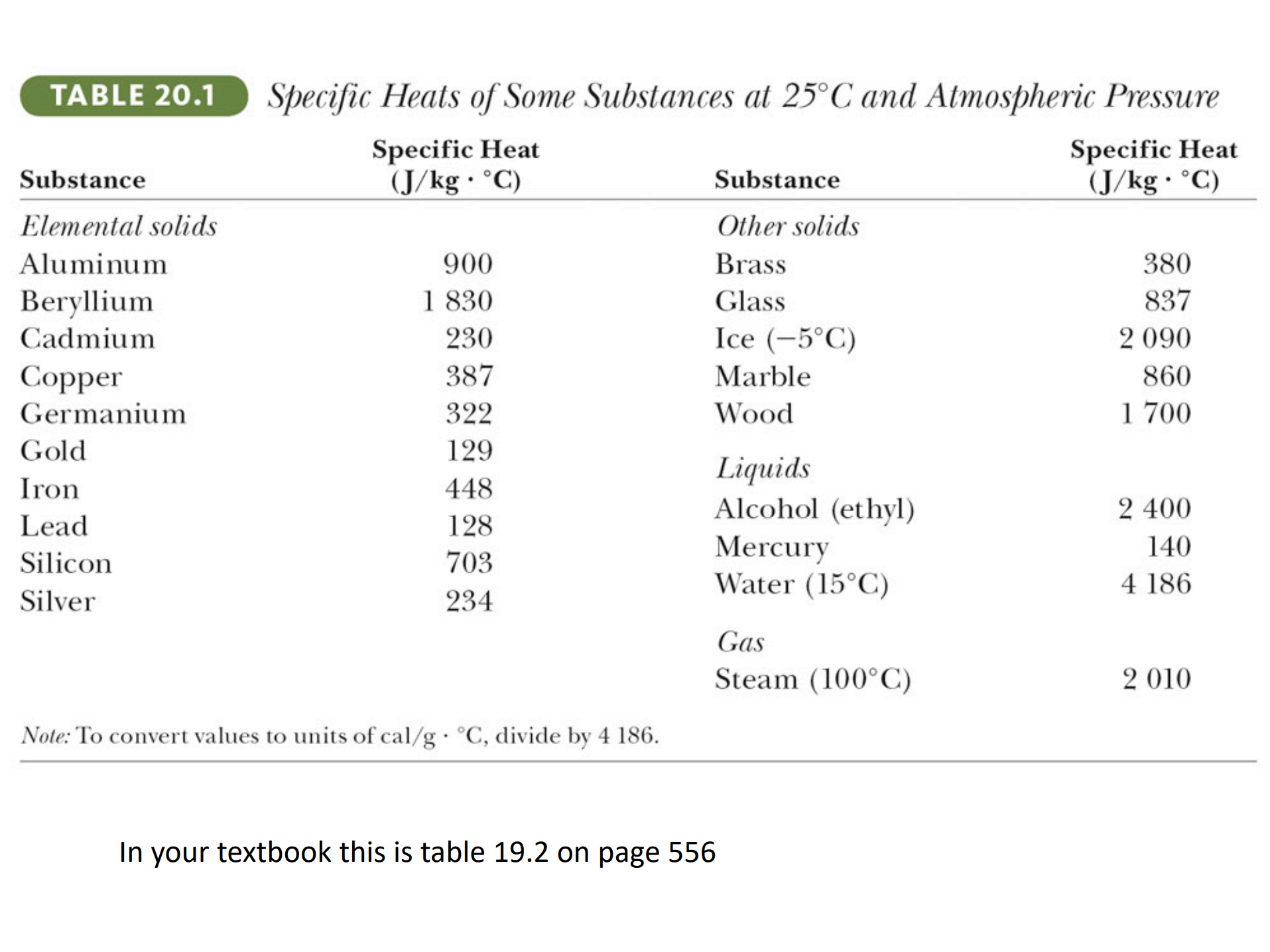
Heat Capacity
Monday, 7 May 2018
4:48 PM
Symbol: ![]()
The amount of energy needed to raise a sample by ![]()
Specific Heat is the heat capacity per unit mass c=C/m![]()
![]()
(Heat capacity)
![]()
Specific heat can be regarded as the insensitivity of a substance to the change of energy.
[ Resistance to change!]