Equipartition of Energy
Sunday, 6 May 2018
7:23 PM
Equipartition - each direction of the motion of a molecule stores on average the same amount of energy
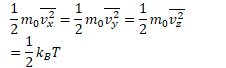
(for each particle)
Degrees of Freedom
Every type of molecule has a certain number of degrees of freedom, expressed as .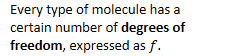
This number represents the independent ways in which the molecule can store energy.
Each degree of freedom allows the molecule to store energy.![]()
Monatomic (1 atom)
- Can move in the x, y, z direction - can have KE in each of these directions (translational)
- Can
rotate and have KE but very little as - mass is concentrated in the nucleus (m)
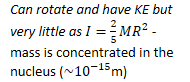
Therefore: 3 degrees of freedom, and ![]()
Diatomic Molecules (2 atoms)
- Translational
motion (x, y, z) 3 degrees of freedom

- Rotational motion
around 2 axes 2 degrees of freedom
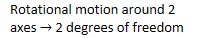
- Vibrational motion
when possible extra 1 degree

Therefore 5/6 degrees of freedom, and ![]()
Polyatomic Molecules (many atoms)
- Translational
motion (x, y, z) 3 degrees of freedom

- Classically can rotate about 3 axes
- Up to 15 degrees of freedom
Total Energy of a Gas
![]()
ie in a monatomic gas of N particles: ![]()