Adiabatic Processes
Monday, 7 May 2018
4:26 PM
An adiabatic process is one which no energy enters nor leaves a system as heat.
![]()
Recall the first law of thermodynamics
![]()
If , then ![]()
In order to change the internal energy of an adiabatic system, work must therefore be done.
// (Q+W)=W
Minimising heat transfer
- Insulate the container
- Perform the process very quickly
- So that the gas reaches a new equilibrium state before there is time for heat to enter the system
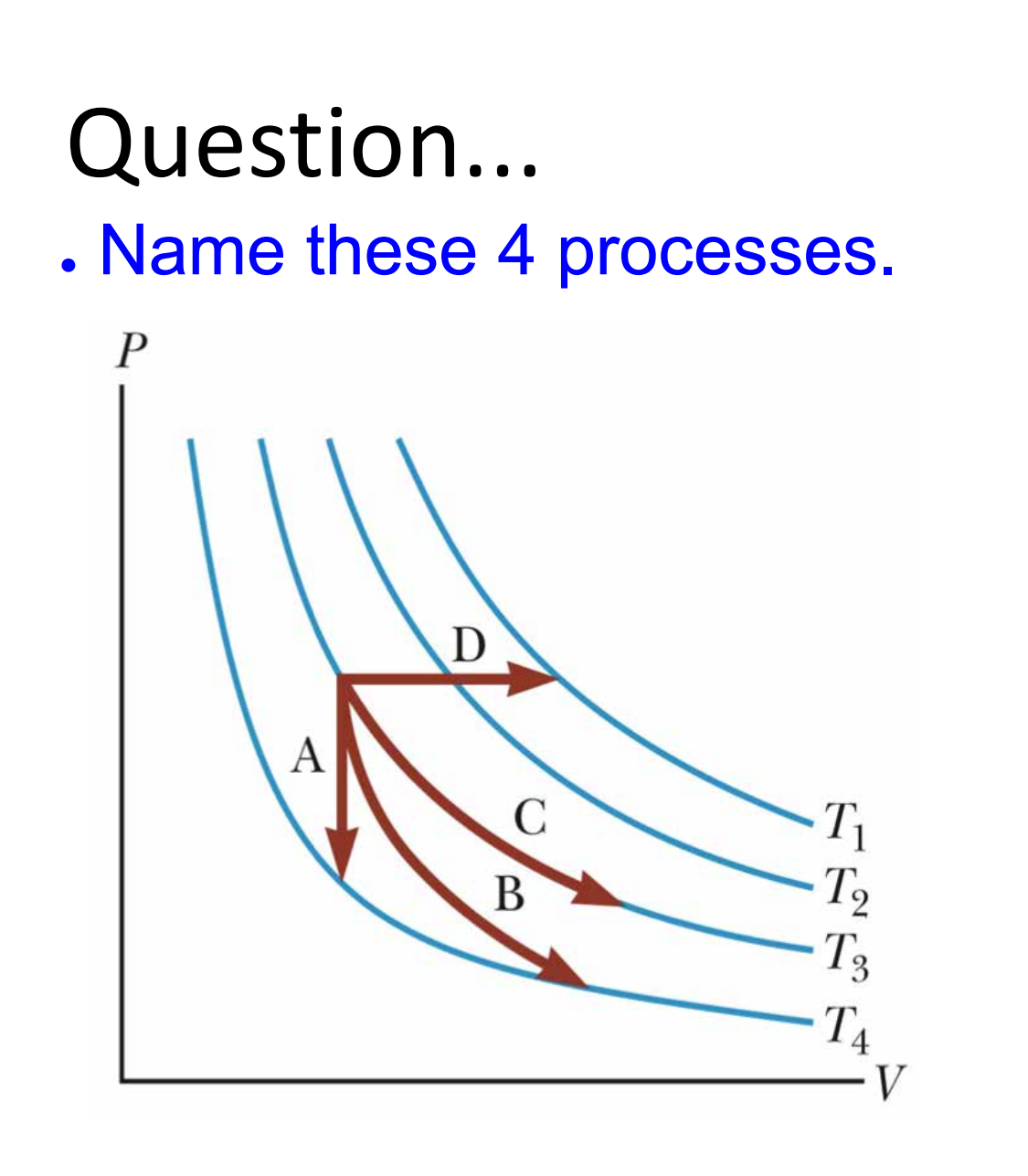
Adiabatic Processes in a P-V Relation
![]()
![]()
![]()
![]()
In an adiabatic process, the pressure, volume and temperature all
change, and are given the relation ![]()
Where
-
pressure![]()
-
volume![]()
-
ratio thingy!?!?!??!![]()
-
constant - constant during the process![]()
Temperature Volume relation
![]()
Isothermal
- constant temperature
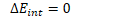
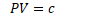
- Any energy that enters the system by heat but leave the system by work
Isobaric
- constant pressure
- Work is generally non-zero as the volume changes
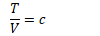
- Heat is generally non-zero as the temperature changes as work is done
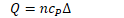
Isovolumetric
- Constant volume
- No work is done as volume does not change
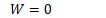
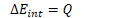
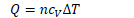
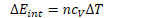
Adiabatic (Q=0)